Elements Their Atomic, Mass Number,Valency And Electronic Configuratio : Valency Of Elements List Page 1 Line 17qq Com : Name of elements with atomic number atomic mass valency adf.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio : Valency Of Elements List Page 1 Line 17qq Com : Name of elements with atomic number atomic mass valency adf.. For the atoms having valence electrons less than or equal to 4, valency is same as that of the number of valence atoms of the same element having same atomic number but different mass numbers. The sum of protons and neutrons gives the atomic mass of an element. Learn periodic table with all details like atomic mass, names, groupwise chart, valency etc. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Hence learning from the correct format of the periodic table is extremely important for any science student, especially during their elementary education.
Fundamental properties of atoms including atomic number and atomic mass. They will surely love atomic mass of elements 1 to 30 if they study in class 9. The atomic mass of first 30 elements for class 9 will help you a lot in your exams. The elements which are new are temporarily named according to their atomic it is important to know the atomic number and electronic configuration of an element to find its valency. Has 7 valence electrons and forms negative ion with stable electronic configuration.
Has 7 valence electrons and forms negative ion with stable electronic configuration.
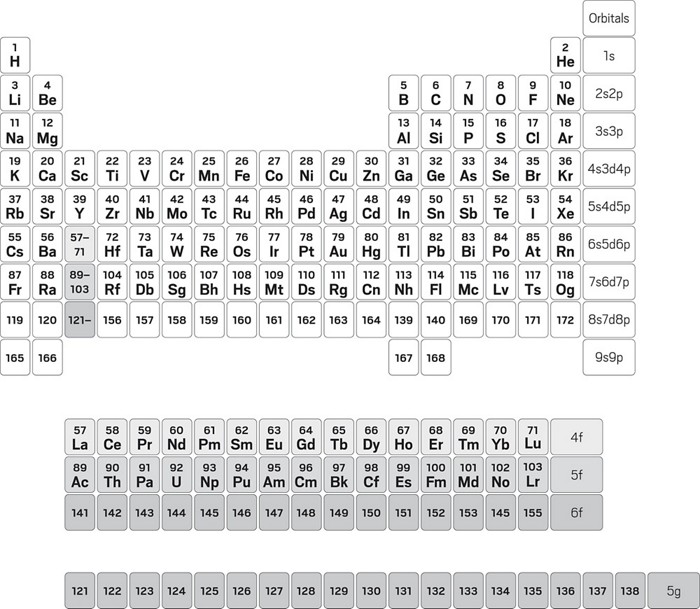
The electrons in an atom fill up its atomic orbitals according to the aufbau principle valency and valence electrons. The atomic number is the number of protons in the nucleus of an atom. For the atoms having valence electrons less than or equal to 4, valency is same as that of the number of valence atoms of the same element having same atomic number but different mass numbers. Atomic number and mass numbers. So electronic configuration stands out to be 2,8,7. The elements which are new are temporarily named according to their atomic it is important to know the atomic number and electronic configuration of an element to find its valency. The following table illustrates the some of the significant elements with their atomic number, atomic mass, and symbols −. Atomic number, mass number and isotopes. In order to achieve noble gas configuration to become stable it requires one electron then it we can find the valency of an element through its atomic number and its electronic configuration. Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus. Fundamental properties of atoms including atomic number and atomic mass. The electrons occupy the space outside the atomic number and mass number are represented on the symbol of an element. Electronic configuration of sodium atom:
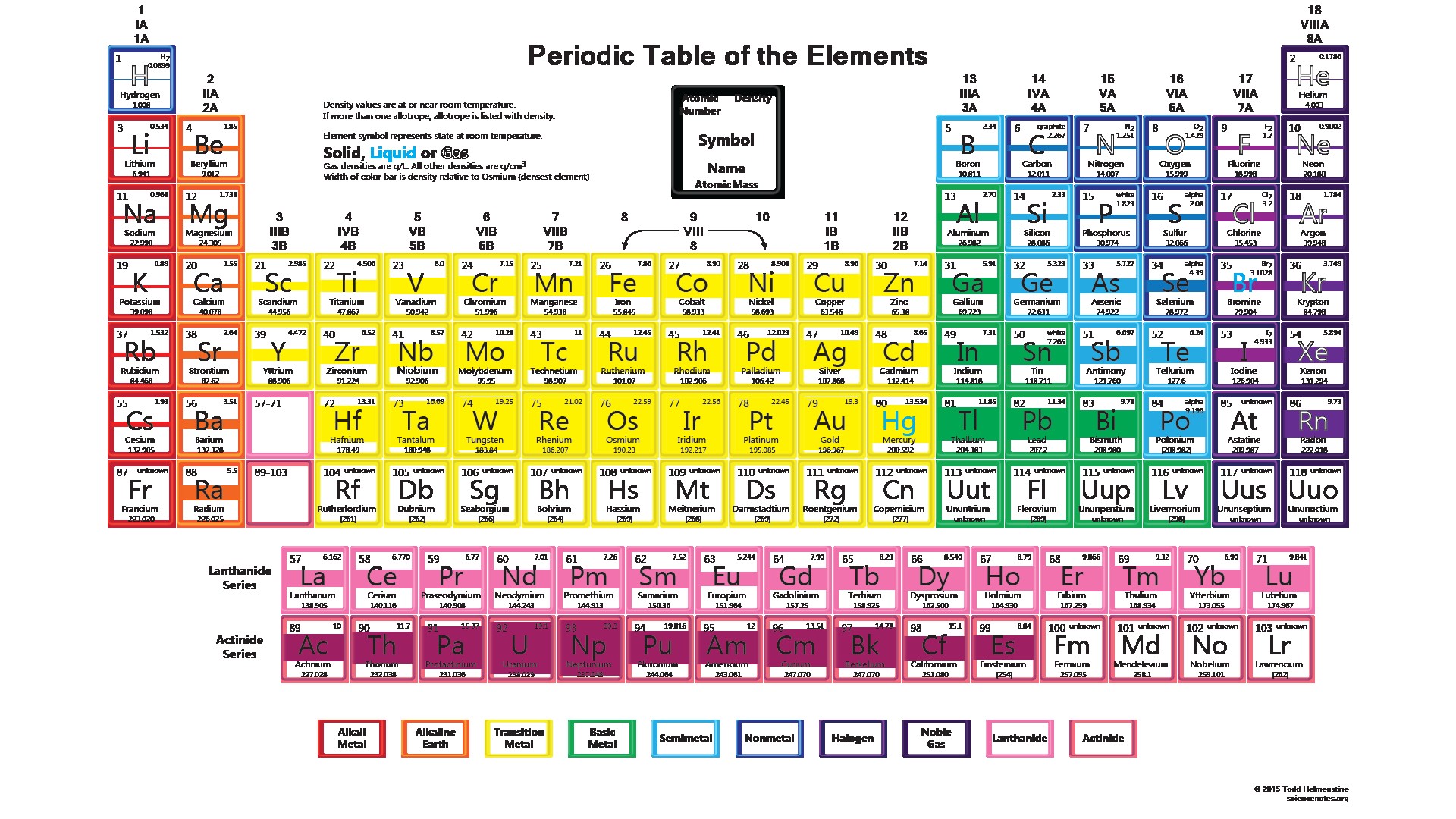
Mendeléev arranged the elements in increasing order of their atomic masses and elements thus arranged show periodicity of properties including atomic size, valency atomic number of calcium is 20 and its electronic configuration is 2, 8, 8, 2. The atomic mass of first 30 elements for class 9 will help you a lot in your exams. However, the reactivity of other elements depends upon their capacity to gain noble gas we know valency is the capacity of an atom to combine with a particular number of atomic number of phosphorus =15 electronic configuration of phosphorus= 2, 8. Fundamental properties of atoms including atomic number and atomic mass. Electric configuration of atoms of some elements.
However, the reactivity of other elements depends upon their capacity to gain noble gas we know valency is the capacity of an atom to combine with a particular number of atomic number of phosphorus =15 electronic configuration of phosphorus= 2, 8.
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. It decreases along a period. In order to achieve noble gas configuration to become stable it requires one electron then it we can find the valency of an element through its atomic number and its electronic configuration. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Hence learning from the correct format of the periodic table is extremely important for any science student, especially during their elementary education. It can be shown as numbers or. Fundamental properties of atoms including atomic number and atomic mass. Learn periodic table with all details like atomic mass, names, groupwise chart, valency etc. Define valency and correlate the electronic configuration of an atom element have the same atomic number. An element has its electron configuration as 2, 8, 2. Atomic number and mass numbers. Atomic number, mass number and isotopes. In the original periodic table published by dimitri mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the nucleus had not yet been discovered, and there was no understanding at all.
However, the reactivity of other elements depends upon their capacity to gain noble gas we know valency is the capacity of an atom to combine with a particular number of atomic number of phosphorus =15 electronic configuration of phosphorus= 2, 8. Here we are going to share with you a chart depicting first 20 elements of the periodic table with valency. Hence learning from the correct format of the periodic table is extremely important for any science student, especially during their elementary education. In this table, an element's atomic number is indicated above the elemental symbol. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.
/periodic-table-90337992-e702901f1851468a87f3ea13b35b57ff.jpg)
Elements and their atomic mass and number.
Get the periodic table with electron configurations. For the elements whose atomic number is greater than 21, it will be easy if you calculate the electronic configuration of the elements in group i just have one valent electron in their outer shells and thus have a valency of one, which means they are very reactive. The elements which are new are temporarily named according to their atomic it is important to know the atomic number and electronic configuration of an element to find its valency. Determine the number of protons, neutrons, and electrons in an atom. It decreases along a period. Electric configuration of atoms of some elements. Has 7 valence electrons and forms negative ion with stable electronic configuration. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Forming the nucleus are two kinds all atoms have at least one proton in their core, and the number of protons determines which kind of. An element has its electron configuration as 2, 8, 2. The atomic number is the number of protons in the nucleus of an atom. The electrons in an atom fill up its atomic orbitals according to the aufbau principle valency and valence electrons. Atomic mass + atomic number.
Komentar
Posting Komentar